
ALIsens® is a patented 3D in vitro alveolar lung model that recreates the human alveolar–capillary barrier under air–liquid interface (ALI) conditions. By combining human epithelial, endothelial, and immune cell lines in a structured architecture, the model enables realistic exposure of inhalable substances such as aerosols, vapors, and particulates.
ALIsens® fits seamlessly into early R&D decision-making, hazard identification, and regulatory-oriented safety packages. It supports compound prioritization, formulation optimization, and the generation of robust data aligned with evolving regulatory expectations, including REACH and OECD-driven frameworks.
ALIsens® combines human lung and immune cell lines in a structured 3D architecture that mimics the alveolar–capillary barrier. Test substances are delivered under controlled air–liquid interface conditions, replicating real inhalation exposure and enabling precise assessment of irritation and sensitization responses.
From start-to-finish, the design and strategy team provide all of the guidance and expertise necessary to build a high-conversion website.
Generate human-relevant, mechanistically informed data designed to support regulatory dossiers as respiratory sensitization moves toward formal requirements.
Identify problematic compounds early and reduce attrition by detecting respiratory hazards before significant R&D and scale-up investments are made.
Detect irritation or sensitization liabilities early in development, minimizing the risk of post-market withdrawals, litigation, or brand damage.
Access a human cell–based alternative aligned with animal testing bans, internal policies, and ethical commitments, without compromising scientific relevance.
The irritation model is the most streamlined configuration of ALIsens®, designed for rapid assessment of local respiratory effects. It focuses on early inflammatory and cytotoxic responses following realistic inhalation exposure. This variant is typically used for screening, formulation comparison, and dose-ranging studies where fast, reliable irritation data are required to guide early development decisions.
Ideal for early-stage hazard flagging and compound ranking
Shorter timelines and lower complexity
Clear indication of irritation potential under ALI conditions

The sensitization model is the most advanced and biologically complex configuration of ALIsens®. By integrating multiple immune-relevant endpoints, it enables the identification of respiratory sensitizers and their differentiation from simple irritants. This variant is intended for decision-critical programs where understanding sensitization risk is essential.
Differentiates respiratory sensitizers from irritants
Designed for regulatory-oriented hazard assessment
Suitable for high-impact compounds and advanced development stages.

Some substances present significant technical challenges due to reactivity, instability, or exposure constraints. The hard-to-test variant of ALIsens® is specifically adapted for such compounds, including anhydrides and other difficult chemistries. Exposure strategies and handling procedures are customized to ensure data quality without compromising model integrity.
Tailored protocols for reactive or unstable substances
Adapted exposure and dosing strategies
Enables assessment of compounds often excluded from standard assays

For organizations with strict animal-free policies or vegan product positioning, ALIsens® is available using a bovine-free culture medium. This option maintains the scientific performance of the model while aligning with internal compliance, ethical commitments, and product claims.
Free of bovine-derived components
Compatible with animal-free and vegan policies
Same study design and outputs as standard ALIsens® models


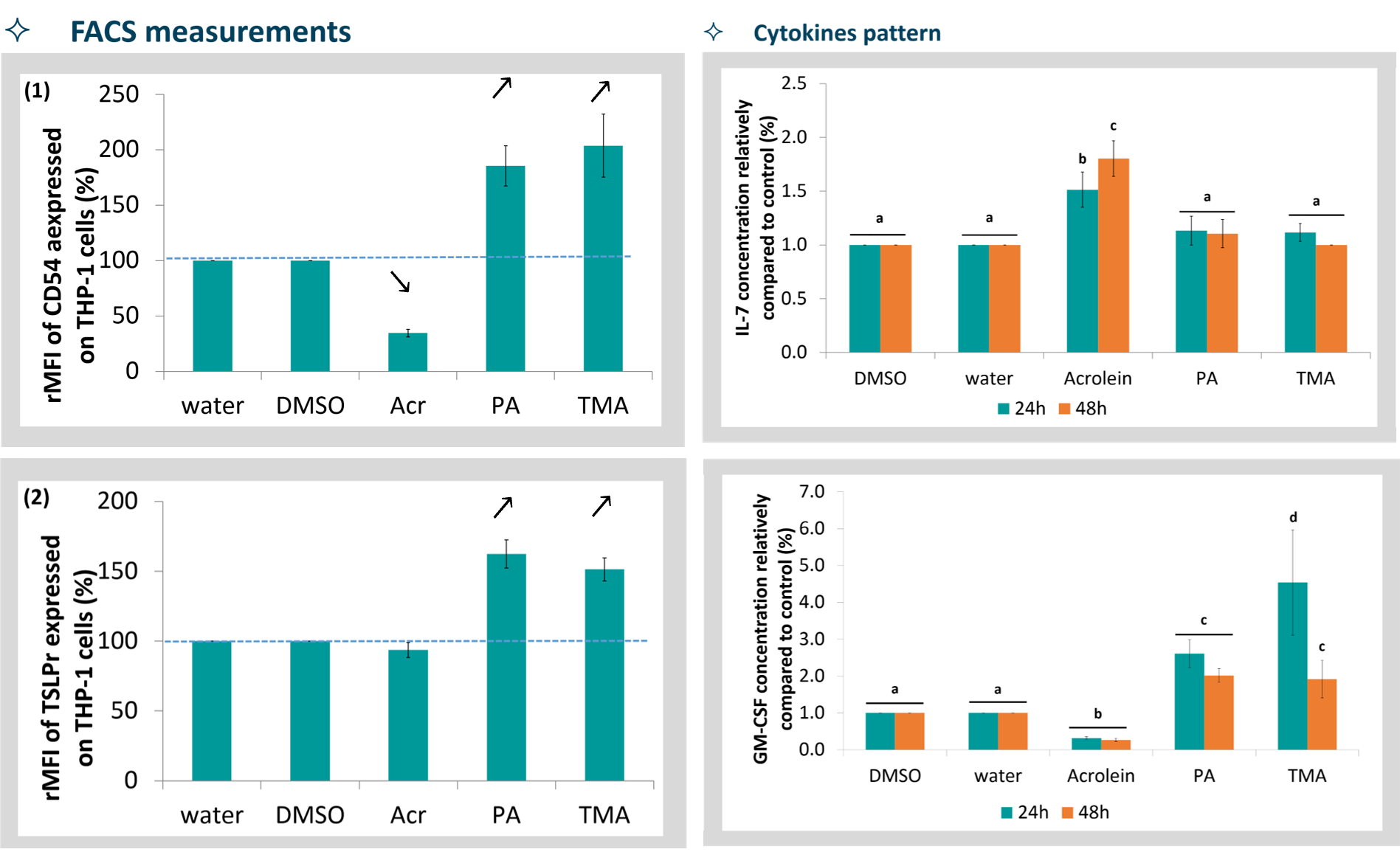

ALIsens® correctly identifies respiratory sensitizers (e.g., phthalic anhydride, trimellitic anhydride) and distinguishes them from irritants (e.g., acrolein) by analysing biomarker expression patterns under controlled ALI exposure.
This capability has been highlighted in independent research showing that ALIsens® can successfully discriminate respiratory sensitizers from skin sensitizers and non-sensitizers — a key challenge for traditional assays.

I have been impressed with Sprocket Rocket since my initial conversation with Miles. He took the time to listen to exactly what I needed and asked great questions. Given my tight timeline, I particularly appreciated his ability to provide me with a scope of work, timeline and budget quickly.
Mark Robinson
Product Designer, Google
I have been impressed with Sprocket Rocket since my initial conversation with Miles. He took the time to listen to exactly what I needed and asked great questions. Given my tight timeline, I particularly appreciated his ability to provide me with a scope of work, timeline and budget quickly.
Mark Robinson
Product Designer, Google
I have been impressed with Sprocket Rocket since my initial conversation with Miles. He took the time to listen to exactly what I needed and asked great questions. Given my tight timeline, I particularly appreciated his ability to provide me with a scope of work, timeline and budget quickly.
Mark Robinson
Product Designer, GoogleExpand respiratory toxicology capabilities y generate human-relevant evidence for client submissions.
De-risk compounds early in development and support regulatory safety assessments for chemicals, pharma, cosmetics and consumer products.
Advance understanding of respiratory sensitization mechanisms and validate new markers and models in inhalation toxicology