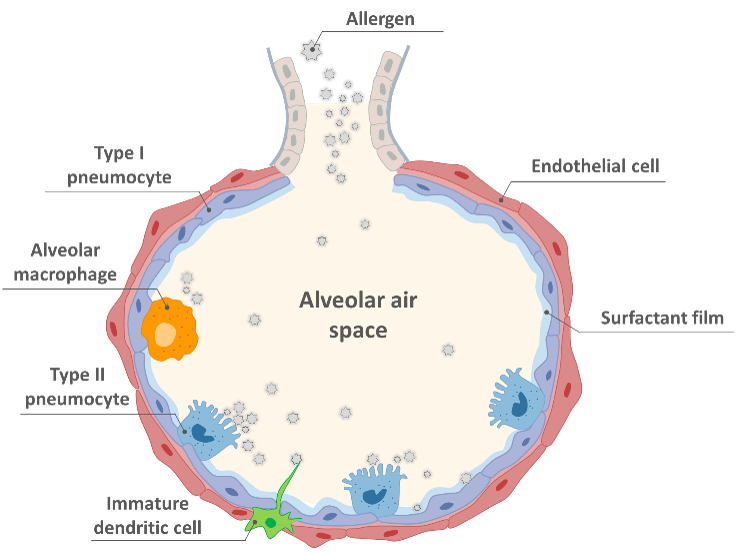
Learn how ALIsens® reproduces the human alveolar barrier under realistic exposure conditions and enables regulatory-grade identification of respiratory sensitizers using a patented, human-relevant in vitro model.
Integrate ALIsens® into your service portfolio and deliver human-relevant, regulator-trusted respiratory sensitization data to your clients.

Respiratory sensitization is a critical but largely unaddressed toxicological endpoint. No validated in vitro methods exist today, while regulatory pressure is increasing and industrial clients are actively requesting reliable, human-relevant data.
ALIsens® was developed specifically for regulatory use. It is based on immortalized human cell lines, aligned with OECD requirements, and is actively progressing through the OECD standardisation pathway for future regulatory acceptance.
Most CROs offer irritation or general inhalation toxicology. Very few can address respiratory sensitization. Today, only one in vitro model is technically designed to reach OECD acceptance, creating a clear differentiation opportunity.
Working with Invitrolize allows CROs to enhance their respiratory toxicology capabilities without increasing internal complexity. Our in vitro models support earlier risk identification, reduce uncertainty for clients, and reinforce your position as a scientifically advanced partner.
The result is stronger client confidence, differentiated services, and improved support for regulatory-facing projects.
Respiratory sensitization is moving from a scientific gap to a regulatory requirement. ALIsens® was designed from the outset for regulatory applicability, using immortalized human cell lines and reproducible endpoints compatible with OECD expectations. The model is progressing toward OECD standardization, positioning CROs to respond early to upcoming REACH, ECHA, EPA, regulatory-driven demands rather than reacting once requirements become mandatory.

Most CROs currently provide respiratory irritation or general inhalation toxicology, which does not address sensitization. Respiratory sensitization remains largely uncovered due to the lack of suitable in vitro methods. Today, only one in vitro model is technically designed to reach OECD acceptance for this endpoint. Early adopters gain a clear competitive edge in tenders, regulatory studies, and long-term framework agreements.

ALIsens® enables CROs to offer respiratory sensitization testing without committing years to internal assay development, method optimization, or biological validation. The model is delivered as a ready-to-use, standardized system with defined protocols, allowing rapid integration into existing in vitro toxicology workflows. This shortens time-to-market dramatically and avoids R&D risk while adding a highly specialized endpoint to the portfolio.

Within the next 2–3 years, respiratory sensitization testing is expected to become widely demanded worldwide as regulatory expectations crystallize. CROs that adopt ALIsens® early can establish market leadership at the national or regional level, build internal expertise ahead of competitors, and become the reference provider before the market becomes crowded. This is a time-limited opportunity to secure first-mover advantage.
Respiratory sensitization testing addresses high-value regulatory and R&D questions for industries dealing with inhalable compounds, including chemicals, cosmetics, pharmaceuticals, tobacco, fragrances, and consumer products. These studies command premium pricing due to their regulatory relevance, complexity, and lack of alternatives. For CROs, this translates into high-margin services with strong repeat demand and low-price sensitivity.






Before onboarding, invitrolize performs a structured technical assessment to confirm that the CRO can reproduce ALIsens® data at regulatory grade.
Together with your team, we define:
Cell culture infrastructure and quality systems.
ALI exposure capabilities (e.g. Vitrocell® or equivalent).
Biosafety level and handling procedures.
Experience with immunological and molecular endpoints.
Data integrity and reporting workflowsThe nature of the compound or formulation.
This step prevents downstream variability and protects regulatory credibility.

Once validated, invitrolize delivers hands-on, method-specific training to the CRO’s scientific staff.
The training includes:
ALIsens® model biology and scientific rationale.
Insert handling and preparation.
ALI exposure protocols for different compound classes.
Endpoint selection and interpretation.
Common failure modes and quality controlshuman respiratory cells to the test compound.
The training is delivered by the original developers of the model.

The CRO executes a predefined set of verification runs using reference compounds.
Objectives:
Confirm inter-lab reproducibility.
Validate signal discrimination between sensitizers and irritants.
Ensure correct execution of exposure and readoutsA comprehensive scientific report.
All results are reviewed jointly with invitrolize. By this, you will get a formal confirmation you can generate data equivalent to invitrolize’s internal benchmarks.

After successful verification, the CRO is formally authorized to offer ALIsens® testing services.
Partner status includes:
The CRO retains full ownership of client relationships and reporting.
Invitrolize provides an exciting opportunity to assess challenging chemicals for challenging endpoints in respiratory toxicity with human-relevant approaches. They are grounded in science while having a clear view of regulatory application to advance toxicology, and we are excited to work with them.
Kristie Sullivan, MPH, Vice President for Research Policy



Learn how ALIsens® reproduces the human alveolar barrier under realistic exposure conditions and enables regulatory-grade identification of respiratory sensitizers using a patented, human-relevant in vitro model.

Answers to the most common technical and regulatory questions before starting a study with Invitrolize.
Still have questions?
Talk directly with our scientific team to discuss your compound, your regulatory context, and the most appropriate study design.
Yes. Respiratory sensitization is increasingly recognized as a major regulatory and safety gap. Industrial clients are already requesting data, particularly for REACH compliance, SSbD strategies, and internal decision-making. Demand is expected to accelerate significantly as regulatory expectations become clearer.
ALIsens® assesses the respiratory sensitizing potential of substances by mimicking the human alveolar barrier under air–liquid interface conditions. It measures immune-relevant cellular responses using a defined set of mechanistic endpoints that allow differentiation between respiratory sensitizers and irritants.
ALIsens® is suitable for:
Low molecular weight (LMW) chemicals
High molecular weight (HMW) substances, including proteins
Complex mixtures
Volatile and poorly soluble compounds
Aerosols and nebulized substances
This broad applicability is a key advantage over many existing in vitro models.
Most existing lung models are designed for irritation, inflammation, or general toxicity and are often based on primary cells. ALIsens® is specifically designed to address respiratory sensitization and is based on immortalized human cell lines, enabling reproducibility and suitability for regulatory standardisation.
Yes. ALIsens® was designed from the outset for regulatory applicability. The model architecture, cell line selection, endpoints, and standardization strategy align with OECD expectations, making it suitable for use in regulatory-oriented studies rather than exploratory research only. The process has been started in January 2026.
ALIsens® data can support regulatory submissions under REACH and related ECHA frameworks, as well as EPA decision-making processes in the US. It is particularly relevant for weight-of-evidence approaches, SSbD strategies, and future respiratory sensitization requirements.
Standard in vitro toxicology infrastructure is sufficient. Requirements typically include:
Cell culture facilities
Air–liquid interface exposure system (e.g. Vitrocell® or equivalent)
Standard molecular and immunoassay tools
No proprietary or highly specialized equipment is required.
Training is focused and method-specific. CRO staff typically require hands-on training covering insert handling, exposure protocols, endpoint execution, and quality controls. After training and verification runs, teams are fully autonomous in routine testing.
ALIsens® is more complex than simple monoculture assays but comparable to advanced 3D or ALI-based in vitro models already used by experienced CROs. The method is standardized, well-documented, and designed for routine execution once implemented, not for continuous optimization.