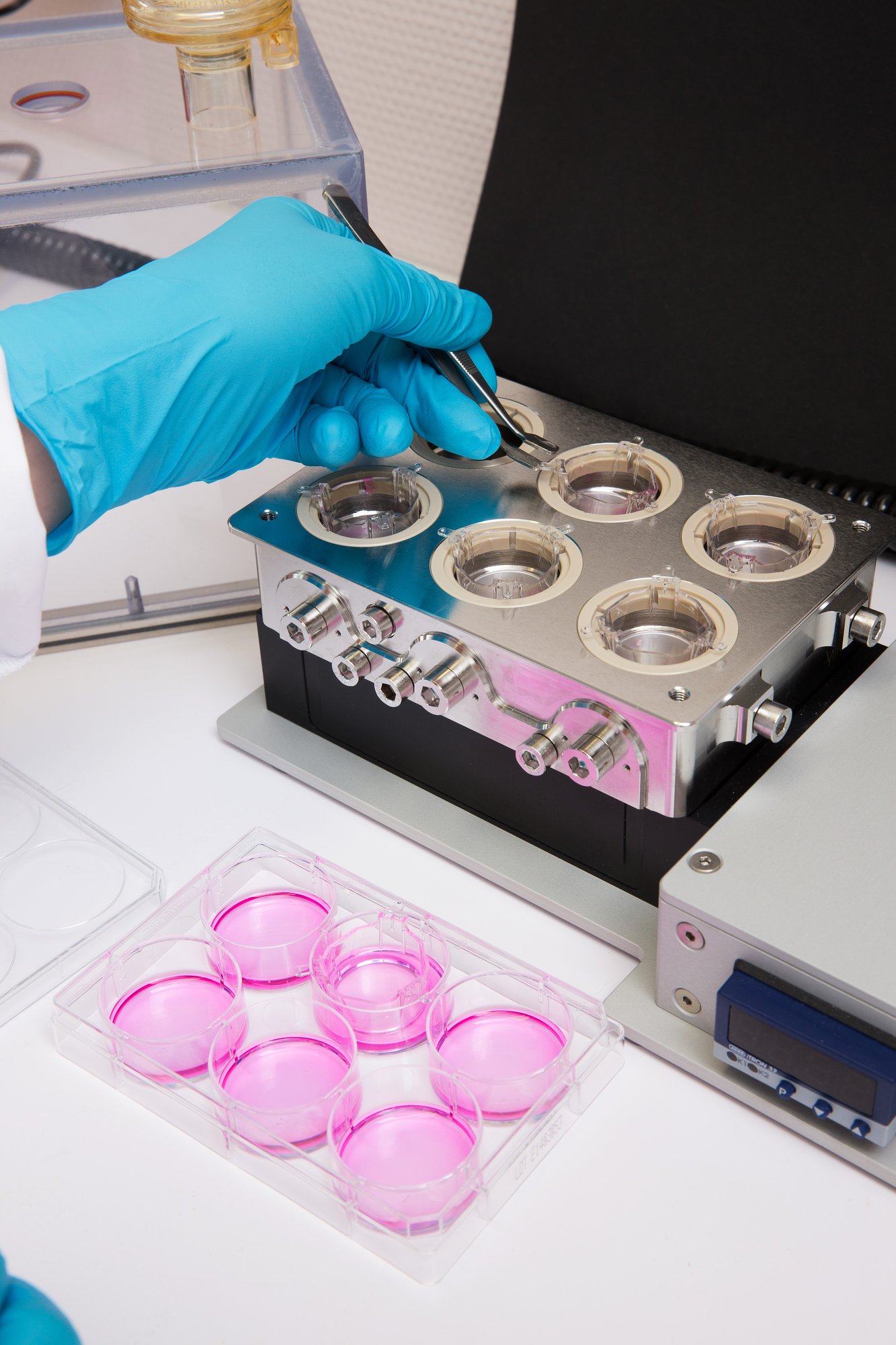
Respiratory sensitization is increasingly scrutinized and expected to become a formal regulatory requirement. Yet industry still lacks validated, human-relevant tools to address this endpoint with confidence.
The Structural Problem
Respiratory sensitization is a recognized health and regulatory concern, yet no validated in vitro method exists to address it. And industry is expected to manage it with no delays.